Home >> Nuclear, worked solutions, binding energy
calculations involving loss of mass
information
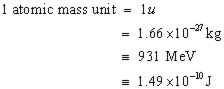
Example #1
Calculate the binding energy(in MeV) of an alpha particle from the following info. :
(ans. to 1 d.p.)
mass of a proton = 1.0076 u
mass of a neutron = 1.0090 u
mass of an alpha particle = 4.0028 u
1 u = 931 MeV
mass of particles in alpha particle = 2 protons + 2 neutrons
= 2(1.0076) + 2(1.0090) = 4.0332 u
mass loss(mass defect) = 4.0332 - 4.0028 = 0.0304 u
since 1 u = 931 MeV,
binding energy = 0.0304 x 931 = 28.3 MeV
Answer: binding energy of an alpha particle is 28.3 MeV
Example #2
Calculate the total energy released(in MeV) when uranium 238 decays by alpha emission to Thorium 234?
uranium 238 = 238.0508 u
thorium 234 = 234.0436 u
alpha partice = 4.0026 u
1 u = 931 MeV
mass loss(mass defect) = 238.0508 - (234.0436 + 4.0026) = 0.0046 u
since 1 u = 931 MeV,
energy released(MeV) = 0.0046 x 931 = 4.3
Answer: energy released from uranium 238 decay is 4.3 Mev
this week's promoted video
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
