Home >> Nuclear, nuclear fission
energy |
Energy from Fission
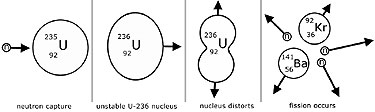
Nuclear fission is the disintegration of a large nucleus(the parent)into two smaller(daughter) nuclei by the capture of a 'slow' (thermal) neutron.
The equation describes the fission of uranium-235 by a slow neutron into barium and krypton nuclei, with the emission of three fast neutrons.
![]()
Energy(200MeV/fission), mostly in the form of kinetic energy of the fragments and gamma ray radiation, is released as a result of a loss of mass in the reaction. This 'lost mass'(about 0.1%) is converted to energy as described by Einstein's mass-energy equation: E=mc2.
In more detail, the average binding energy per nucleon of the products is greater than that of the parent nucleus.
From the graph, the binding energy per nucleon increases from about 7.4 to 8.8 MeV.
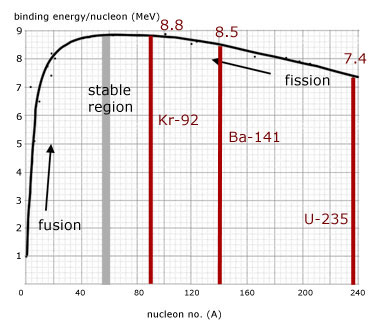
total binding energy of parent nucleus uranium-235
= no. nucleons x binding energy per nucleon
= 235 x (7.4 ) = 1739.0
total binding energy of fragments, barium-141 + krypton-92
= no. nucleons x binding energy per nucleon
= 141 x (8.5) + 92 x (8.8 ) = 2008.1
difference in total binding energy(and hence energy released)
= 2008.1 - 1739.0 = 269.1 MeV (approx)
A more accurate value for the fission yield/reaction is 215Mev.
We can get an idea now of the energy released when a small mass of U-235, say 10 kg, undergoes fission.
235 g of U-235 contains 6.02 x 1023 atoms.
(The mass number of an element expressed in grams contains the Avagadro Number of atoms.)
The number of atoms in 10 kg of U-235 is :
(atoms per kg) x (10 kg)
= (6.02 x 1023/0.235 kg) x (10kg)
= (6.02 x 1024) / (2.35 x 10-1)
= 2.56 x 1025 atoms
total energy release = (no. U-235 atoms)x(energy per fission)
= 2.56 x 1025 x 215 = 5.5 x 1027 Mev
As 1 MeV = 106 x 1.6 x 10-19 = 1.6 x 10-13 Joules,
the total energy released is :
1.6 x 10-13 x 5.5 x 1027 = 8.8 x 1014J
or 880,000,000,000,000 J
Compare the burning of coal: energy/kg = 3.5 x 107 J/kg
10 kg of coal therefore produces 3.5 x 108 J
1 kg U-235 produces 2,514,000 times the energy of 1 kg coal.
1 kg U-235 produces approx. the same energy as burning 2,514 tonnes of coal.
Nuclear Reactor Design
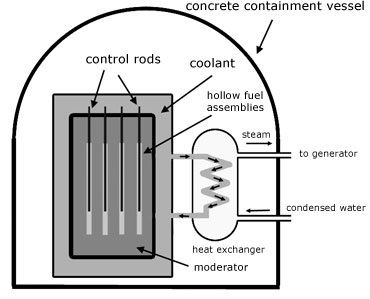
The neutrons created by fission are fast. Infact they are too fast to be captured by other U-235 nuclei to maintain fission. For capture to occur, the neutrons must be slowed down. The material used to do this is called a moderator. Common examples are carbon(graphite) and heavy water(deuterium dioxide D2O .
Ideally fission should procede at the rate of one fission for one neutron produced. The trouble is that in the process two and sometimes three neutrons are produced.
To limit the numbers of neutrons in the reactor control rods are used. These are rods of boron-coated steel which absorb neutrons. When the rods are raised from the reactor, neutron numbers increase. When they are lowered, neutron numbers decrease. In this way the rate of fission is controlled.
The heat energy produced by the reactor must eventually be used to produce steam to drive turbines. The water used for this purpose cannot be used to cool the reactor, otherwise the turbines would themselves become radioactive and the escaped steam would pollute the atmosphere.
So what is needed is an intermediary fluid called the coolant. This can be a liquid or a gas. Examples are: carbon dioxide, pressurized water, liquid sodium. The coolant circulates around pipes carrying water in an arrangement called a heat exchanger.
The whole reactor is encased in a large containment vessel made of thick concrete. This is for security to protect the core against aircraft crashes and terrorism. The concrete shield is also to prevent the escape of harmful radiation to protect workers and the environment.
The Chain Reaction
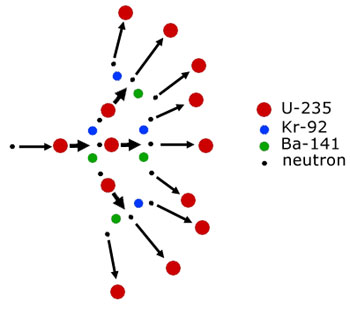
A chain reaction in broad terms means one that is self sustaining. In other words it carries on by itself without any further external intervention.
Chain reactions occur both in nuclear reactors and atomic (fission) bombs. For a fission reaction to continue a uranium-235 nucleus(or other fissionable material) must at the very least emit one neutron to initiate fission in another nucleus.
This level of reaction of one neutron/nucleus is the approximate level used in nuclear reactors. However, in atomic bombs all the available neutrons per nucleus (max. 3) are used to trigger more fissions. A run-away fission reaction ensues.
In a reactor, this set of circumstances is called a melt-down. So much heat is generated that the actual core melts. This gives rise to what is called the China Syndrome(also a film, Michael Douglas). The melted reactor is so hot it melts its way out of the containment vessel, down into the rocks below. If you live in the USA it would melt itself through the crust in the direction of China, on the other side of the globe. Of course in reality, before going much further the melt would meet ground water. This would create a titanic explosion and contaminate a vast area of the surrounding countryside (note: Chernobl disaster 26th April 1986).
There is a minimum amount of mass for fission to be maintained called the critical mass. Less than this amount, the fission reaction will die out for lack of neutrons. For uranium-235 the critical mass is approximately 15 kg.
The size of the critical mass is governed to some extent by the loss of neutrons from the surface. When the mass is encased in a 'neutron reflector' material (eg lead, graphite, steel) to contain the neutrons, the critical mass can be made smaller.
Artificial Nuclides
These are man-made nuclei that have a short half-life and give out a required type of radioactivity. Artificial nuclides are made in reactors, where there is a plentiful supply of neutrons. Exposure to neutrons makes nuclei larger(neutron rich) and unstable. So radioactivity occurs to redress the balance and make nuclei more stable again.
Another way of altering the nucleus is by firing particles at it in a particle accelerator. In this way artificial elements(those with Z >92) were first created eg Curium, Einsteinium, Californium, Fermium etc.
The whole process of changing one nucleus into a different nucleus is called artificial transmutation.
The radioactivity and radiation from radionuclides can be measured and imaged in a variety of situations.
uses:
nuclear medicine - diagnosis,treatment of cancer, genetics, biochemistry, - use of radioactive 'tags' called tracers to follow chemical processes and flow and to highlight targeted areas for imaging.
technetium-99m, cobalt-60, strontium-90, caesium-137
industry - monitoring of flow, corrosion, cracks, rate of wear
potassium-40, thorium series, uranium series
arms industry - tank armour, armour piercing shells
depleted uranium
ionisation detectors - smoke alarms
americium-241
this week's promoted video
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
