Home >> Thermal, thermometers
liquid in glass |
||
Liquid in glass
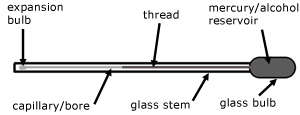
The thermometer works by an expanding liquid in a vacuum, moving against a scale.
There are a number of disadvantages to this instrument:
1.) The glass itself expands and contracts and leading to under and over reading of temperatures.
2.) Parallax errors mean readings are only 0.1oC accurate.
3.) The diameter of the bore is not consistent.
4.) Their large thermal capacity means that they do not react quickly and they may affect the temperature they are trying to measure.
This is how the two liquids used in thermometers, alcohol and mercury compare:
alcohol
- transparent, must be used with a dye
- heat conduction poor
- sticks to glass - concave meniscus
- temperature range ~ 150oC ... -114.9 oC
mercury
- opague
- is a metal and therfore a good heat conductor
- does not stick to glass - convex meniscus
- temperature range~ 356oC ... -39oC
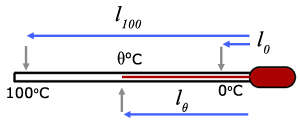
The temperature on a liquid in glass thermometers can be calculated by making certain measurements.
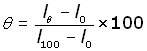
Thermocouple
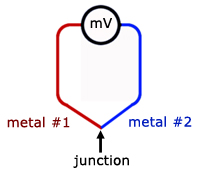
Thermocouples work on a principle called the thermoelectric or Seebeck Effect.
When two different metal wires are twisted together at a junction, an EMF(electromotive force) is generated across the loose ends. The magnitude of this EMF relates to the temperature at the junction.
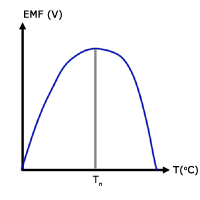
On the graph, the value of T where the EMF is maximum is called the 'neutral temperature'. The gradient (d(EMF)/dT) anywhere on the curve is called the thermoelectric power .
Measured with a high resistance millivoltmeter, EMF values are in the range 1mV - 4mV/100oC.
A more convenient and efficient setup is to have two junctions instead of one, but still have just two metals. The reference cold temperature is usually melting ice.
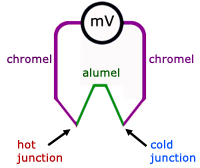
Typical pairs of metals and temperature ranges:
|
metals |
temperature rangeoC |
| chromel/alumel |
~1100 max. |
| Pt/Pt-Rh |
1100 - 1700 |
| Fe/Constantan |
95 - 760 |
| Cu/Constantan |
200 - 350 |
Resistance thermometer
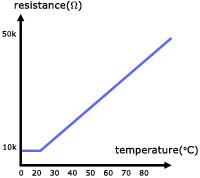
The property of metals that their resistance is temperature-dependent makes them ideal as thermometers. The metal of choice is platinum as a result of its high melting point(1773oC) and large resistance temperature coefficient*.
*α(alpha) a big increase in resistance for a small rise in temp.
In practice resistance thermometers are either thin films of platinum on a substrate or platinum wire wound around a former.
Thermistor
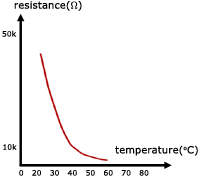
Semiconductors like metals have resistance that is temperature-dependent. So they too make ideal thermometers.
The difference is, as temperature rises, the resistance of metals increases, but the resistance of semiconductors decreases.
Semiconductors have large resistance temperature coefficients, but they are negative. This means that there is a big decrease in resistance for a small rise in temperature.
Typical temperature range of thermistors is -70oC ... 300oC .
The thermal capacities of thermistors are small. So they absorb little heat energy and do not appreciable affect the temperature they are measuring.
Thermistor resistance is ~1kΩ .
Constant-volume gas thermometer
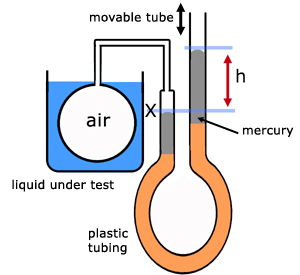
In its original state the glass bulb is full of air and the mercury levels are the same. A mark(X) is made against the glass to record this.
When the bulb is placed in a hot liquid for a temperature reading, the air in the bulb expands, pushing the mercury down on the left and up on the right.
To get the air in the flask back to its original volume, the movable tube is lowered until the mercury is at the level previously marked.
There is now a level difference(head) h between the two tubes. This is a measure of the pressure of the gas without taking account of atmospheric pressure pA. So accounting for atmospheric pressure , the pressure pθ of the gas at temperature θ is:
![]()
note, all pressures expressed in mm of mercury
It follows that the temperature of the gas, θ is given by:
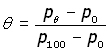
where p0 and p100 are pressures at 0oC & 100oC respectively.
Temperatures using the constant-volume gas thermometer can be measured to two decimal places. However there are several sources of error that prevent further accuracy:
1. the capillary tube air is not heated
2. the volume of the bulb increases with temp.
3. air is not an 'ideal gas'
[ About ] [ FAQ ] [ Links ] [ Terms & Conditions ] [ Privacy ] [ Site Map ] [ Contact ]
